Chem 1 Ionic Vs Molecular Properties Review Continued
2.1.1: Comparing Ionic and Molecular Substances
- Page ID
- 225663
- Know the physical properties of ionic and molecular substances.
The physical state and properties of a particular compound depend in large part on the type of chemical bonding it displays. Molecular compounds, sometimes called

covalent compounds, display a wide range of physical properties due to the different types of intermolecular attractions such as different kinds of polar interactions. The melting and boiling points of molecular compounds are generally quite low compared to those of ionic compounds. This is because the energy required to disrupt the intermolecular forces between molecules is far less than the energy required to break the ionic bonds in a crystalline ionic compound (Figure \(\PageIndex{1}\)) . Ionic solids typically melt at high temperatures and boil at even higher temperatures. For example, sodium chloride melts at 801 °C and boils at 1413 °C. (As a comparison, the molecular compound water melts at 0 °C and boils at 100 °C.). The water solubility of molecular compounds is variable and depends primarily on the type of intermolecular forces involved.
Figure \(\PageIndex{1}\) Interactions in Ionic and Covalent Solids.
Since molecular compounds are composed of neutral molecules, their electrical conductivity is generally quite poor, whether in the solid or liquid state. In solid form, an ionic compound is not electrically conductive because its ions are unable to flow ("electricity" is the flow of charged particles). When molten, however, it can conduct electricity because its ions are able to move freely through the liquid (Figure \(\PageIndex{2}\); Video \(\PageIndex{1}\)).
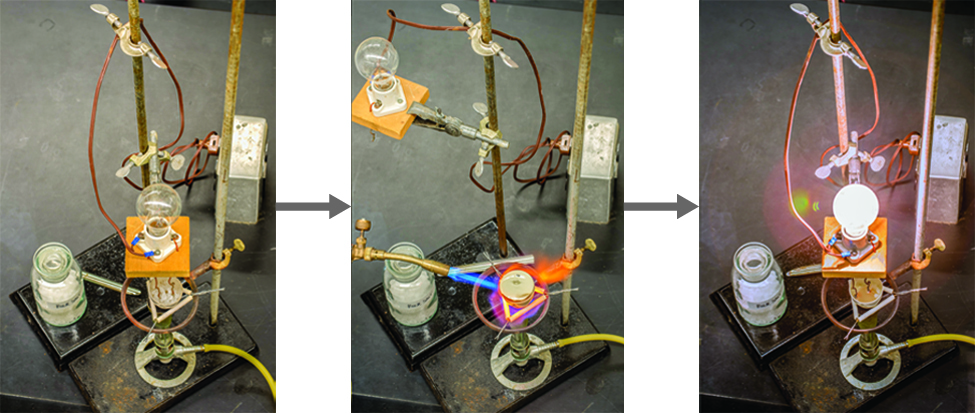
Conductivity of Molten Salt
Video \(\PageIndex{1}\) Watch this video to see a mixture of salts melt and conduct electricity.
| Table \(\PageIndex{1}\) Comparison of Ionic and Molecular Compounds | ||
|---|---|---|
| Property | Ionic Compounds | Molecular Compounds |
| Type of elements | Metal and nonmetal | Nonmetals only |
| Bonding | Ionic - transfer of electron(s) between atoms | Covalent - sharing of pair(s) of electrons between atoms |
| Representative unit | Formula unit | Molecule |
| Physical state at room temperature | Solid | Gas, liquid, or solid |
| Water solubility | Usually high | Variable |
| Melting and boiling temperatures | Generally high | Generally low |
| Electrical conductivity | Good when molten or in solution | Poor |
One type of molecular compound behaves quite differently than that described so far. A covalent network solid is a compound in which all of the atoms are connected to one another by covalent bonds. Diamond is composed entirely of carbon atoms, each bonded to four other carbon atoms in a tetrahedral geometry. Melting a covalent network solid is not accomplished by overcoming the relatively weak intermolecular forces. Rather, all of the covalent bonds must be broken, a process that requires extremely high temperatures. Diamond, in fact, does not melt at all. Instead, it vaporizes to a gas at temperatures above \(3500^\text{o} \text{C}\).
Summary
- The physical properties of a material are affected by the intermolecular forces holding the molecules together.
- Ionic compounds usually form hard crystalline solids with high melting points. Covalent molecular compounds, in contrast, consist of discrete molecules held together by weak intermolecular forces and can be gases, liquids, or solids at room temperature and pressure.
- Ionic compounds in molten form or in solution can conduct electricity while molecular compounds do not..
Source: https://chem.libretexts.org/Courses/Willamette_University/WU%3A_Chem_199_-_Better_Living_Through_Chemistry/02%3A_Chemicals_in_Water/2.01%3A_Types_of_Bonds/2.1.01%3A_Comparing_Ionic_and_Molecular_Substances
0 Response to "Chem 1 Ionic Vs Molecular Properties Review Continued"
Post a Comment